[ad_1]
Scientists in South Korea have developed a extremely soluble, steady natural redox-active molecule to be used in aqueous redox stream batteries. The newly developed naphthalene diimide (NDI) molecule affords a better storage capability than current vanadium gadgets.
Redox stream batteries are one of the vital promising applied sciences for large-scale stationary storage functions because of their low capital price, low combustion, and lengthy lifetime of greater than 20 years. Nonetheless, for the reason that value of vanadium, essentially the most broadly used energetic materials for redox stream batteries, has elevated in recent times, scientists are actively searching for redox supplies to exchange them. this.
Now a analysis group from the Korea Superior Institute of Science and Know-how (KAIST) and Pohang College of Science and Know-how (POSTECH) in South Korea has developed a soluble and steady energetic molecule – naphthalene diimide (NDI) . It’s used rather than vanadates in aqueous stream batteries.
Though NDI molecules are nearly insoluble in water and subsequently little has been investigated, the South Korean analysis crew was capable of bind 4 ammonium compounds and obtain a solubility of as much as 1.5M in water. By regulating the π–π interactions of those natural molecules, the researchers prevented any extreme facet reactions and diminished cyclability that would have been attributable to radical formation throughout the electron switch course of. .
“We demonstrated the ideas of molecular design by modifying an current natural energetic molecule with low solubility and utilizing it as an energetic molecule for redox stream batteries,” stated Professor Hye Ryung Byon. “Now we have additionally proven that in a redox response, we are able to use molecular interactions to suppress the chemical reactivity of the radical-forming molecules.”
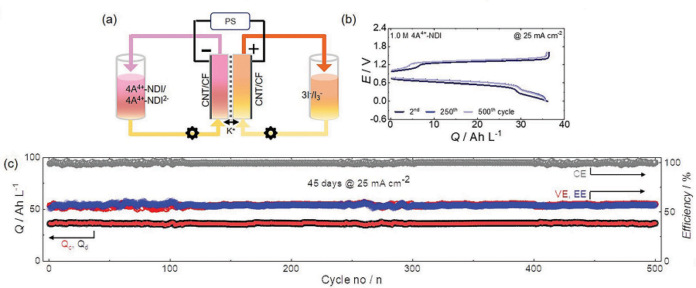
As well as, the researchers confirmed that when a 1M NDI resolution was utilized in impartial redox stream batteries for 500 cycles, 98% of its capability was maintained. This implies 0.004% capability decay per cycle, and solely 2% of its capability might be misplaced if the battery is operated for 45 days.
The researchers additionally confirmed that the developed NDI molecule can maintain two electrons per molecule, which means that 2M electrons could be saved for each 1M NDI resolution used.
For reference, the vanadium utilized in vanadium redox stream batteries, which require a extremely concentrated sulfuric acid resolution, has a solubility of about 1.6M and may solely maintain one electron per molecule, which implies it may well retailer a complete of 1.6 M electrons. Subsequently, the newly developed NDI energetic molecule exhibits a better storage capability in comparison with the present vanadium gadgets.
“It needs to be used later for aqueous redox stream batteries, with excessive vitality density and excessive solubility, it additionally has the benefit of being utilized in impartial pH electrolytes,” stated Ryung Byon. “Vanadium redox stream batteries in the present day use acidic options, which trigger corrosion, and we hope that our molecule will clear up this subject. Because the current lithium ion-based ESS is susceptible to burning, we have to will develop safer and cheaper next-generation ESS, and our analysis exhibits nice promise in fixing this.
The researchers focus on their findings within the paper “Controlling π-π interactions in extremely soluble naphthalene diimide derivatives for impartial pH aqueous redox stream batteries.,” which was just lately printed in Superior Supplies.
This content material is protected by copyright and is probably not reused. If you wish to cooperate with us and need to reuse a few of our content material, please contact: [email protected].
[ad_2]
Source link